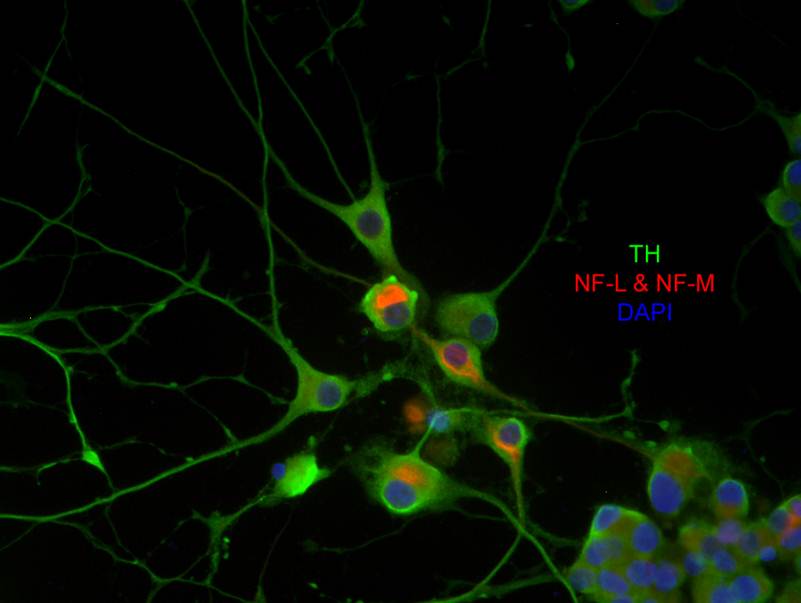
ResearchOur main research program focuses on the cellular mechanisms underlying neuronal damage in neurodegenerative disorders such as Parkinson's disease (PD). We are analysing the role of some oxidative and inflammatory agents in cellular degeneration and mortality. In this context, we are also interested in neuroprotective cellular mechanisms, i.e. the possibility that some steroids, particularly phytoestrogens and phytosterols, may act as antioxidative and/or anti-inflammatory molecules. Even if oxidative reactions are normal features of cellular metabolism, the production of oxidative molecules can overwhelm cellular antioxidative capacity, causing oxidative stress. Recent studies support the existence of a link between oxidative stress and the complex neurodegeneration processes. Thus, we have developed a neuronal cell culture system to assess the oxidative power of molecules such as the herbicide paraquat and MPP+, one of its structural analogues associated with the induction of PD. We have demonstrated that these environmental toxins act as neurodegeneration mediators. Hyperglycemia is now recognised as a cause of oxidative stress in the brains of diabetic patients, potentially leading to neurodegeneration. In fact, the incidence of neurodegenerative disorders, such as Parkinson's and Alzheimer's disease, is admittedly more elevated in diabetic patients. In light of this epidemiological evidence, our research program also hinges on the assessment of neurodegeneration in high-glucose conditions, with a particular interest for dopaminergic neurons. We have established a model of hyperglycemia in cultivated dopaminergic neurons to delineate the precise oxidative mechanisms by which elevated concentrations of glucose provoke cell death. As an extension to this in vitro work, we are also interested in investigating the vulnerability of dopaminergic pathways in the brains of long-term hyperglycemic rodents. |
We have evaluated several strategies to protect neurons and prevent neurodegeneration by reducing oxidative stress. It is well-known that estrogen replacement therapies in postmenopausal women reuduce the risk of developing several neurodegenerative diseases. However, recent clinical trials have revealed that these therapies are linked with secondary hormonal effects. Interestingly, phytoestrogens are naturally-occurring molecules that may exert anti-oxidative actions in vivo and in vitro and protect against neurodegenerative diseases without causing secondary hormonal events. Our results show that some phytoestrogens, such as reservatrol and quercertin, reduce neuronal mortality induced by environmental toxins and hyperglylcemia.
Microglial activation and neuroinflammation are also associated with PD pathogenesis. Indeed, cytokines are suspected to play a role in the dopaninergic neuron apoptosis observed in PD. The development of our neuroinflammation model has helped us to demonstrate the anti-inflammatory role of phytoestrogens. Finally, our experiments on the cellular targets of oxidative and inflammatory stress in nerve cells may open doors to the rational design of neuroprotective anti-oxidant and anti-inflammatory agents with decreased hormonal side-effects. The techniques deployed in our laboratory include the culture of neurons, astrocytes and microglial cells, western blotting, immunofluorescence, RNA interference as well as real-time polymerase chain reaction. Occasionally, and only if necessary, we use laboratory animals. |