| PPH-1003, Modélisation moléulaire |
|
PPH-1003, Modélisation moléculaire - Liens et sites
Informations et sites intéressants à consulter
- ACD/Labs
- Amazon.ca
- Atoms in Molecules
- BookFinder.com
- Bristol
- Chemexper - L'assistant IUPAC.
Bienvenue à la page de l'assistant IUPAC. Cette page et les suivantes sont destinées à vous aider dans la nomenclature des composés organiques. Le contenu de ces pages se base sur les principes essentiels de nomenclature organique repris dans l'édition 1979 des règles de la nomenclature IUPAC et dans le livre reprenant les recommandations de 1993. Il y a aussi un logiciel "Expereact WEB" qui permet de stocker localement toutes les informations concernant des produits chimiques et ainsi d'avoir une "mémoire" des produits synthétisés au sein d'un laboratoire. Il est également possible de soumettre certaines données sur un serveur central rendant les donnes publiques (http://ccd.chemexper.com). Le programme est accessible sur : http://www.chemexper.apexhosting.com/xtweb/.
- Chemical information sites indexed at the ChemFinder WebServer
- Chemical of the Week
- ChemSketch 8.0 Freeware
- ChemWeb, The World Wide Club for the Chemical Community
- Chimie générale & organique par Gérard Dupuis - Professeur au Lycée Faidherbe de LILLE
- Chmoogle

- Chromatek
- Computational chemistry at University of North Carolina at Wilmington, including some very interesting lectures
- DCU Molecular Viewing Gallery
- Density Functional Theory - An Introduction Page
- Elemental Discoveries. David Bradley's Elemental Discoveries, the online monthly magazine of chemistry on the web, is now available at , and has been getting an increasing number of subscribers. David's articles appeal to a broad range of chemists and non-chemists, and cover current topics in the chemical sciences with a different focus each month.
- Dimensions fractales
- Glossary of terms for Computational Chemistry
- Hantzsch-Widman Home Page. Revision of the extended hantzsch-widman system of nomenclature for heteromonocycles.
- Hypercube
- Démos
- HyperChem Web Viewer
- Exemples
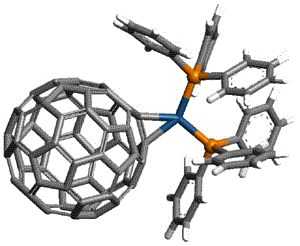
The theory of atoms in molecules was developed by Professor Richard Bader and colleagues at McMaster University, Ontario, Canada. The theory is presented in journal articles and in the monograph, Atoms in Molecules, a Quantum Theory by Richard F. W. Bader (Clarendon Press, 1990). A less mathematical introduction is presented in Atoms in Molecules, an Introduction by Paul Popelier (Prentice Hall, 2000).
This theory is based on the topology of the electron density distribution in molecules. In formaldehyde, for example, the electron density increases towards each nucleus. This can be depicted with contour lines of the electron density and/or with the gradient vector field of the electron density (as shown in class). A gradient vector points in the direction of increasing electron density. The gradient paths are all perpendicular to the contour lines that they cross. Gradient paths terminate at the carbon, oxygen and hydrogen nuclei (the nuclear attractors). Other gradient paths terminate at critical points between the bonded atoms (bond critical points, BCP). Bonded atoms are characterized by bond paths between them that contain a BCP. A bond path is defined by two gradient vectors that originate at the BCP and terminate at one of the nuclei. The BCP is thus a minimum in electron density along the bond path, but the BCP is a maximum in electron density for a line perpendicular to the bond path. A BCP is located on an interatomic surface, which this theory uses to divide the molecule into its constituent atoms (or atomic basins). An interatomic surface (also referred to as a zero-flux surface) does not contain any gradient paths that terminate at nuclei, but instead its gradient paths terminate at a BCP. The magnitude of the electron density at the bond critical point is indicative of the strength of the bond. Another feature of the BCP of interest is the ellipticity (if any) of the electron density around the critical point. AIM atomic charges are calculated by subtracting the total amount of electron density in an atomic basin (found by integration) from the total number of electrons in the isolated atom. There are other features of the AIM theory that we will not go into at this point (e.g., the Laplacian of the electron density). In class we will see how the Gaussian98 program can be used to find bond critical points, the electron density at these points, and the AIM atomic charges.
Note that in formaldehyde there are bond paths with critical points where we expect bonds but not between atoms that we regard as non-bonded.
- IgNobel Prize. The annual Ig®Nobel Prize Ceremony honors individuals whose achievements "cannot or should not be reproduced." Ten prizes are given to people who have done remarkably goofy things by genuinely bemused genuine Nobel Laureates.
- Introduction to computational chemistry, incluant les conversions d'unités suivantes:
- IUPAC Home Page
- Chemistry Java, Javascript and other
- Logiciels
- Advanced Chemistry Development, Inc. CNMR, HNMR: spectrum prediction, ACD Name: IUPAC name generation. Allez sur la page de logiciels gratuits, inscrivez-vous (c'est gratuit) et downloader la dernière version de ChemSketch...
- ChemFinder
- Chemscape Chime: Download. Visitez aussi la MDL Chemscape Chime Sample Page. La FAQ et d'excellentes pages d'exemples à MDLI et UMass par Eric Martz sont disponibles.
- Free ISIS/Draw License for Academic and Personal Use at Home
- RasMol
- MDL® Chemscape and MDL® Chime Pro
- Modèles Moléculaires en format PDB
- Molecular Library at New York University
- Molecule of the day
- Molecule of the Month Project:
- MolviZ.Org
- Oxford
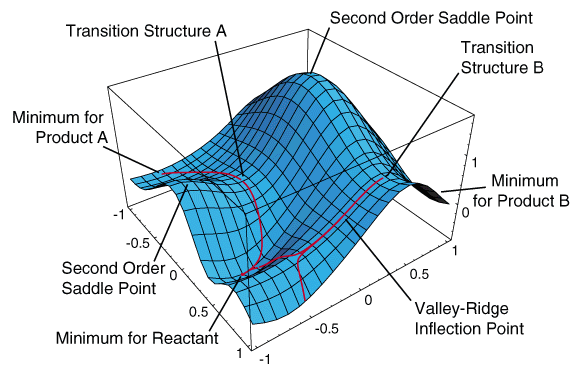
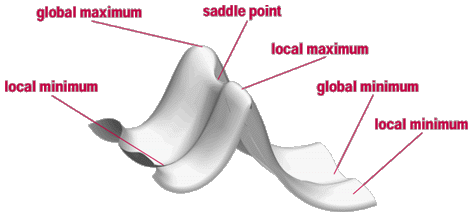
- Potential Energy Surfaces
- The Protein Data Bank
- Protein Explorer: Parmi les façons de visualiser une molécule, on peut soit entrer le PDB id d'une protéine (1LYS par exemple pour le lysozyme du blanc d'oeuf) ou bien entrer l'URL d'un chemin d'accès à un fichier PDB, qui peut aussi bien être sur le web que sur son disque dur. Pour obtenir les fichiers PDB de protéines, vous opuvez utiliser le site web de The Protein Data Bank. Il est aussi recommendé d'aller faire un tour à Downloadable UMass Chime Resource Packages
- PyMOL Home Page: PyMOL is a USER-SPONSORED molecular modeling system with an OPEN-SOURCE foundation.
- Site chimique du mois de l'OCQ
- Dr. Stefan Immel - Simulation de polysaccharides
- Spectroscopie UV-Visible : règles de Woodward-Fieser
- Le Sympathique Chimiste
- Teaching with Chime. Très beau site utilisant Chime pour l'enseignement de la chimie.
- Tesseract et hypercube
- Stereoscopic Animated Hypercube
- Tesseract at Wikipedia, de même que l'article suivant portant sur tous les Polychorons
- Tesseract - from MathWorld
- Tesseract Foldout
- The Tesseract (or Hypercube)
- Théorie, thèses et cours en ligne
- ACCVIP Australian Computational Chemistry via the Internet Project. This has developed from the CAUT Computational Chemistry Project.
- Basic ab initio
- Novel Methods for Large Molecules in Quantum Chemistry by Ross David Adamson
- TURBOMOLE - Program Package for ab initio - Electronic Structure Calculations
- Wavefunction - Spartan
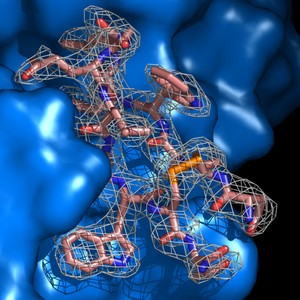
PyMOL is a molecular graphics system with an embedded Python interpreter designed for real-time visualization and rapid generation of high-quality molecular graphics images and animations. It can also perform many other valuable tasks (such as editing PDB files) to assist you in your research.
The extensible core PyMOL module (hosted here at SourceForge) is available free to everyone via the "Python" license (a simple BSD-like permission statement), but we ask all users to purchase a license and maintenance agreement in order to cover our development and support costs.
In order to motivate such sponsorship, we offer support and other incentives to PyMOL licensees with current maintenance subscriptions. In this way, we seek to insure the viability of the Open-Source project by providing a specific incentive (or reward) for outside support. However, our hope is that only a small subset of PyMOL's total value will need to be restricted to Incentive packages -- just enough to justify regular contributions and keep the project self-sustaining.
